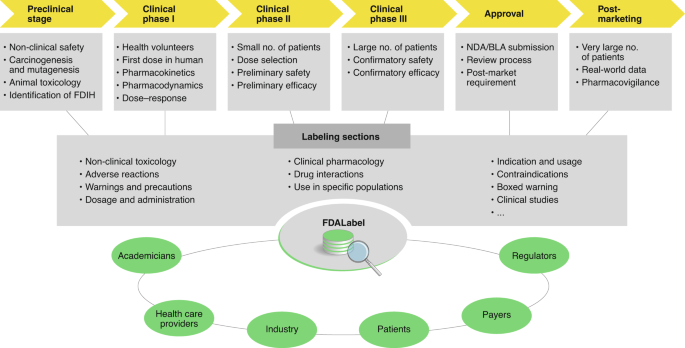
40 fda structured product labels
FDA SPL - Structured Product & Drug Labeling Composition Process Structured Product Labeling (SPL) is a Health Level Seven (HL7) International standard for regulatory guidance documents as a method for communicating product ... Structured Product Labeling (SPL) is a document markup ... - openFDA Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging ...
docs.drugbank.com › csvCSV Format Reference | DrugBank Help Center DrugBank Help Center
Fda structured product labels
Guidance for Industry - Indexing Structured Product Label - FDA Structured Product Labeling. U.S. Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER). What is Structured Product Labeling (SPL)? - Freyr Solutions Structured Product Labeling (SPL) is a standard document issued by Health Level Seven (HL7) to exchange information related to medicinal product and ... Structured Product Labeling Overview SPL files are submitted for drug products manufactured at these facilities. Exchanging SPL Data. DailyMed. FDA-to-NLM - Daily transmissions of up-to-date ...
Fda structured product labels. What is Structured Product Labeling? | SPL Simplified Apr 22, 2022 ... Structured Product Labeling (SPL) is the FDA's adopted standard for communicating product and quality information. › moneywatchMoneyWatch: Financial news, world finance and market news ... Get the latest financial news, headlines and analysis from CBS MoneyWatch. Structured Product Labeling - Wikipedia Structured Product Labeling (SPL) is a Health Level Seven International (HL7) standard which defines the content of human prescription drug labeling in an ... labels.fda.govFDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.)
dailymed.nlm.nih.gov › dailymed › spl-resources-allDailyMed - Download All Drug Labels Full Releases. Warning: The full human prescription and OTC archive files, dm_spl_release_human_rx.zip and dm_spl_release_human_otc.zip, are no longer available due to size considerations. Structured Product Labeling (SPL) Implementation Guide with ... - FDA Structured Product Labeling (SPL). Implementation Guide with Validation. Procedures. Technical Specifications Document. This Document is incorporated by ... › media › 151710August 23, 2021 Approval Letter - Comirnaty - Food and Drug ... Aug 23, 2021 · U.S. Food & Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 Our STN: BL 125742/0 . BLA . APPROVAL . BioNTech Manufacturing GmbH MTHSPL (FDA Structured Product Labeling) Source Information The U.S. National Library of Medicine (NLM) produces the Metathesaurus FDA Structured Product Labels (MTHSPL), which is based on the Food and Drug ...
› industry › fda-data-standards-advisoryStructured Product Labeling Resources | FDA Aug 17, 2022 · The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. › Members_Meeting_DatesIDM Members Meeting Dates 2022 | Institute Of Infectious ... Feb 16, 2022 · IDM Members' meetings for 2022 will be held from 12h45 to 14h30.A zoom link or venue to be sent out before the time.. Wednesday 16 February; Wednesday 11 May; Wednesday 10 August Structured Product Labeling Overview SPL files are submitted for drug products manufactured at these facilities. Exchanging SPL Data. DailyMed. FDA-to-NLM - Daily transmissions of up-to-date ... What is Structured Product Labeling (SPL)? - Freyr Solutions Structured Product Labeling (SPL) is a standard document issued by Health Level Seven (HL7) to exchange information related to medicinal product and ...
Guidance for Industry - Indexing Structured Product Label - FDA Structured Product Labeling. U.S. Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER).




















![Food Labeling 101 - FDA Regulations Guide [2022] | Artwork Flow](https://global-uploads.webflow.com/5f59aa263c234bb74025de57/5fa4f81b6e365340866e0eeb_Inner-Images-4.jpg)



Post a Comment for "40 fda structured product labels"